Abstract

Background:NPM1 and FLT3 are commonly mutated in patients with acute myeloid leukemia (AML). While FLT3 internal tandem duplication (ITD) is known to confer worse prognosis even in the setting of NPM1 according to the recent European LeukemiaNet (ELN) 2022 criteria, the prognostic impact of FLT3 tyrosine kinase domain (TKD) in this population remains unclear. One recent study suggests FLT3-TKD and NPM1 co-mutation defines a highly favorable prognostic group (Boddu et al.). Furthermore, NPM1-mutated AML with both FLT3-ITD and FLT-TKD mutations are rare and not well described.
Objective: In this study, we explored the prognostic impact of FLT3-ITD and/or FLT3-TKD in patients with NPM1-mutated AML.
Methods: This was a multicenter, retrospective study (Moffitt Cancer Center, Weill Cornell, and Memorial Healthcare System) of NPM1-mutated AML patients who were diagnosed and treated from 2013 to June 2022. Inclusion was restricted to NPM1-mutated AML patients who also harbored FLT3-ITD, FLT-TKD, or both (n=107). All patients have next generation sequencing (NGS) performed at diagnosis. Kaplan-Meier, univariate (log-rank), and multivariate (Cox regression) analyses were performed.
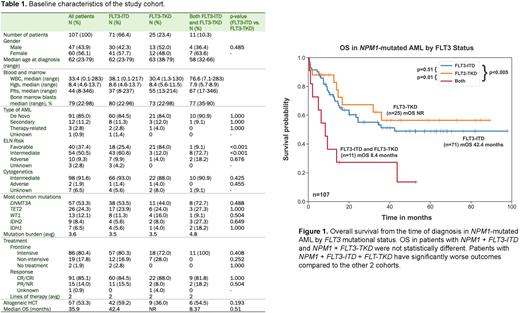
Results: Among 107 patients (47M/60F, median age 62 at diagnosis, range 23-79), 85% (91/107) had de novo AML. By ELN 2017 criteria, 37.4% (40/107) had favorable risk, 50.5% (54/107) had intermediate risk, and 9.3% (10/107) had adverse risk disease. Over 90% had intermediate risk cytogenetics at the time of diagnosis. Common co-mutations included DNMT3A (53.3%), TET2 (24.3%), WT1 (12.1%), IDH2 (8.4%), and IDH1 (6.5%) (Table 1).
There were 66.4% (71/107) of patients with NPM1+FLT3-ITD, 23.4% (25/107) with NPM1+FLT3-TKD, and 10.3% (11/107) with NPM1 and both FLT3-ITD and FLT-TKD (NPM1+FLT3). Patients with NPM1+FLT3-ITD achieved CR/CRi in 84.5% (60/71) similar to patients with NPM1+FLT3-TKD, 88% (22/25), p=1.0. Most patients in both groups received intensive frontline therapy (80.3% vs. 72%, p=0.408). Forty-two (59.2%, 42/71) NPM1+FLT3-ITD patients received allogeneic hematopoietic cell transplant (HCT) compared to 36% (9/25) of NPM1+FLT3-TKD patients, p=0.193. The median overall survival (mOS) was not significantly different between NPM1+FLT3-ITD (42.4 months) and NPM1+FLT3-TKD (not reach), p=0.51. The NPM1+FLT3 cohort had dismal mOS of 8.4 months, which is significantly worse compared to those with NPM1+FLT3-ITD (p<0.005) and NPM1+FLT3-TKD (p=0.01) (Figure 1).
Univariate analysis showed co-occurrence of FLT3-ITD and FLT-TKD significantly impacted OS (HR 2.94, 95% CI: 1.42-6.08, p=0.004). Multivariate analysis using covariates including age over 60, AML type, mutation burden (4 or more), and co-occurrence of FLT3-ITD and FLT-TKD confirmed its prognostic significance on survival (HR 2.96, 95% CI: 1.41-6.20, p=0.004).
Conclusions: Our findings suggest very poor outcomes in patients with NPM1+FLT3 and no significant difference observed in survival between NPM1+FLT3-ITD and NPM1+FLT3-TKD. The prognosis of NPM1-mutated AML is increasingly being refined as more knowledge is gained in the molecular level. More studies are needed to understand the different gene-gene interactions that underlie this unique subset.
Disclosures
Chan:Syntrix Pharmaceuticals: Research Funding. Hussaini:Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Decibio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Diaceutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Guidepoint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Tegus: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Padron:Stemline: Honoraria; Syntrix Pharmaceuticals: Research Funding; BMS: Research Funding; Blueprint: Honoraria; Taiho: Honoraria; Kura: Research Funding; Incyte: Research Funding. Roboz:Astellas: Consultancy; Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; Actinium: Consultancy; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; Jazz: Consultancy, Other: travel; Takeda: Consultancy; Amgen: Consultancy; GlaxoSmithKline: Consultancy; Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; Bristol Myers Squibb: Consultancy; Jasper Therapeutics: Consultancy; Agios: Consultancy, Research Funding; MedImmune: Consultancy, Research Funding; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses; Bayer: Consultancy, Other: Travel and accommodation expenses; Celltrion: Consultancy, Other: Travel and accommodation expenses; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Sandoz: Consultancy, Other: Travel and accommodation expenses; Daiichi Sankyo: Consultancy; MEI Pharma: Consultancy, Research Funding; Otsuka: Consultancy; Bristol Myers Squibb: Consultancy; Roche: Consultancy; Helsinn Therapeutics: Consultancy; Mesoblast: Consultancy; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Array BioPharma: Other: Travel and accommodation expenses; Clovis Oncology: Other: Travel and accommodation expenses; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; Eisai: Other: Travel and accommodation expenses; CTI: Research Funding; Karyopharm Therapeutics: Research Funding; Mofitt Cancer Center: Research Funding; Amgen: Consultancy, Other: travel; Agios: Other: travel, Research Funding; Onconova Therapeutics: Research Funding; Tensha Therapeutics: Research Funding. Desai:Takeda, Bristol Myers Squibb, Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Research: Research Funding. Komrokji:Taiho: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; CTI biopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Honoraria, Other, Speakers Bureau; Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sweet:AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet:Novartis: Consultancy; Jasper Therapeutics: Consultancy; Dedham Group: Consultancy; Agios/Servio: Consultancy; Servier: Consultancy; Boxer Capital: Consultancy; Dava Oncology: Consultancy; Syntrix Pharmaceuticals: Research Funding; Astellas: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy. Sallman:AbbVie: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau; Intellia: Membership on an entity's Board of Directors or advisory committees; Nemucore: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Lixte: Patents & Royalties: LB-100; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal